BioStable Science & Engineering Announces the First Commercial Use of the HAART 200™ Aortic Annuloplasty Device
Austin, TX – September 26, 2017 – BioStable Science & Engineering, Inc. announced today the first commercial use of the HAART 200 Aortic Annuloplasty Device in the U.S. by doctors at the West Virginia University (WVU) Heart and Vascular Institute in Morgantown, West Virginia. Drs. Lawrence Wei, Vinay Badhwar and J. Scott Rankin were the collaborating surgeons for the procedure. The HAART 200 Aortic Annuloplasty Device is the only FDA cleared annuloplasty device designed to facilitate bicuspid aortic valve repair. The WVU Heart and Vascular Institute is the first of a select group of centers expected to participate in a limited release of the product this year.
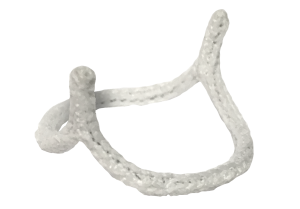
HAART 200 Aortic Annuloplasty Device
“Surgeon interest in the HAART 200 Aortic Annuloplasty Device has been remarkably high because of the potential benefits of valve repair in this young patient population and the technical challenges of existing repair methods,” added Dr. Joe Cunningham, BioStable board member and Managing Director of lead investor, Santé Ventures. “FDA clearance of the HAART 200 Aortic Annuloplasty Device is a tremendous milestone that greatly expands the market opportunity for BioStable in the U.S.”
John Wheeler, President and CEO of BioStable Science & Engineering, concluded by saying, “Valve repair is widely recognized as the treatment of choice for patients with mitral valve disease. We believe the HAART Aortic Annuloplasty Devices will be the technologies that unlock the benefits of valve repair for patients with aortic valve disease and are very pleased to have our full product line (Generic) available in the U.S.”
About BioStable Science & Engineering
BioStable Science & Engineering is a cardiovascular device company focused on developing and commercializing proprietary valve repair technologies that provide an alternative to valve replacement for patients with aortic valve disease. The company’s HAART Aortic Repair Technologies are designed to simplify and standardize aortic valve repair, enabling surgeons to offer the recognized clinical benefits of valve repair to patients undergoing surgical correction of aortic insufficiency or aortic root aneurysm. To learn more visit www.biostable-s-e.com.
The HAART 200 Aortic Annuloplasty Device is investigational and not available for sale.
Contact:
John Wheeler
President & CEO
[email protected]
512-386-1996 x153
