HAART Aortic Valve Repair Technologies
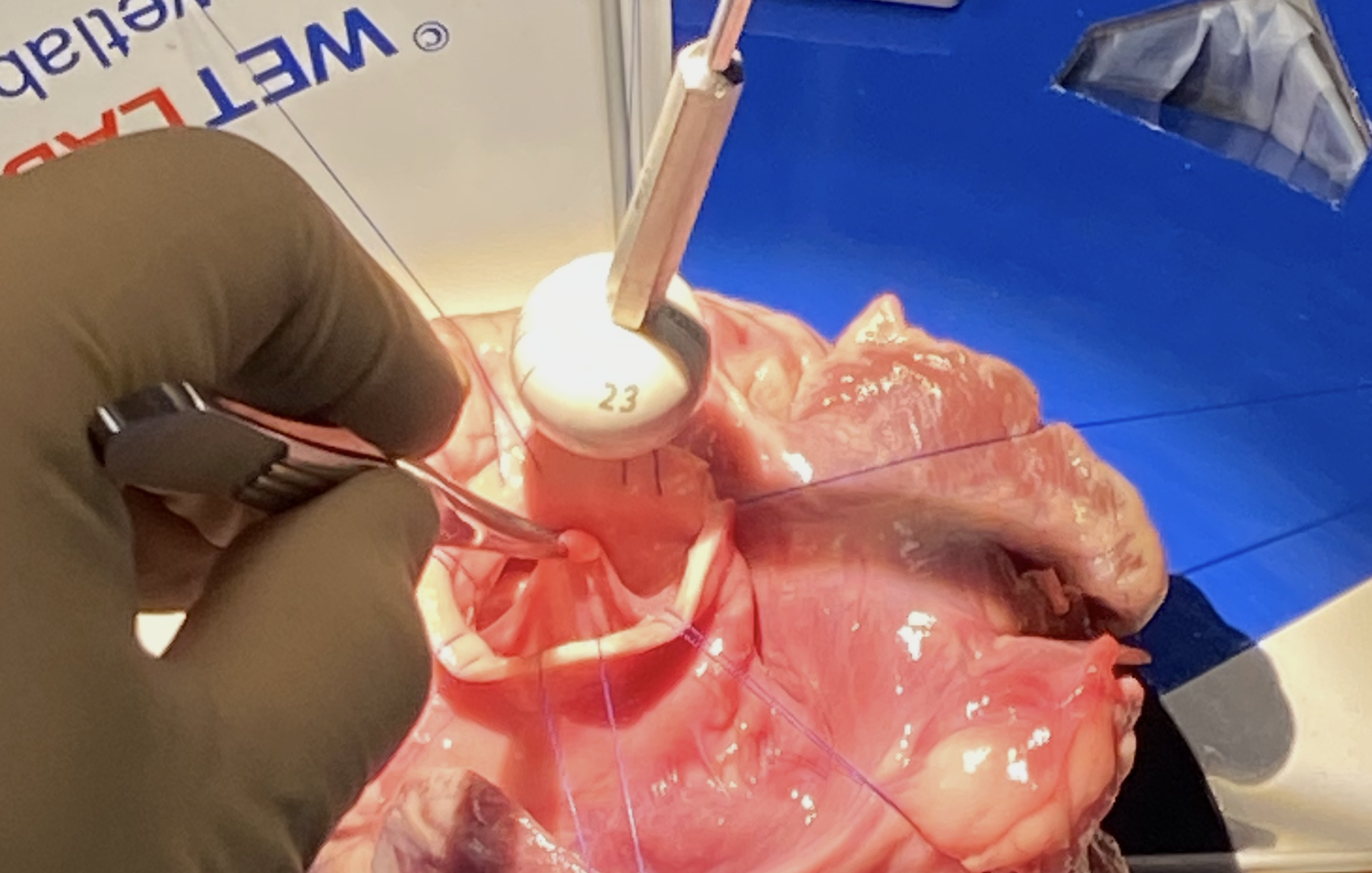
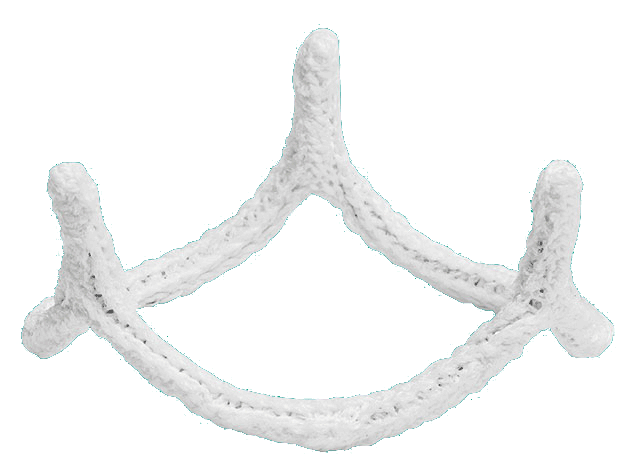
Resize the aortic valve annulus to reduce dilatation
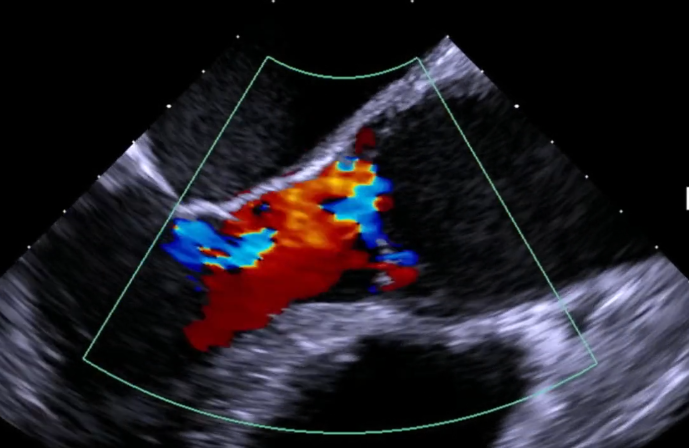
Reshape the aortic valve to restore more normal, three-dimensional coaptation geometry
Stabilize the aortic valve to reduce the risk of recurrent annular dilatation
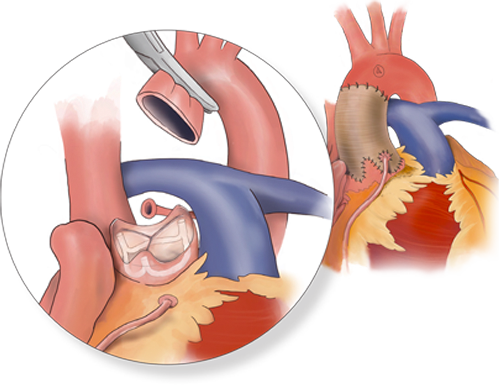
Valve Sparing Root
Replacement Simplified
Restores & Stablizes the Annulus
without Root Mobilization